Project 1: CREATE-NEO
|
As one of ten NIH-funded Centers for Research on Emerging Infectious Diseases (https://creid-network.org/),
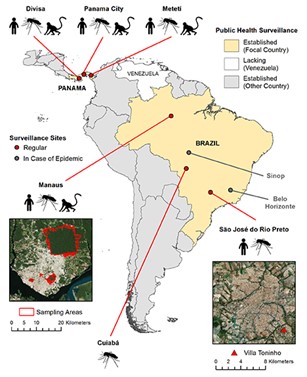
the Coordinating Research on Emerging Infectious Disease Encompassing the Neotropics (CREATE-NEO, https://www.utmb.edu/createneo/ home/create-neo-home) provides a nimble and flexible network of surveillance sites in Central and South America coupled with cutting-edge modeling approaches to anticipate and counter emerging arboviruses. Co-PI’s Nik Vasilakis and Kathryn Hanley partner with a wide array of partners across the US, Brazil and Panama to enact CREATE-NEO’s mission. |
|
Como um dos dez Centros de Pesquisa sobre Doenças Infecciosas Emergentes financiados pelo NIH (https://creid-network.org/), o Coordinating Research on Emerging Infectious Disease Encompassing the Neotropics (Centro de pesquisa em doenças infecciosas emergentes nos Neotrópicos) (CREATE-NEO, https://www.utmb.edu/createneo/home/create-neo-home/) fornece uma rede ágil e flexível de vigilância em diferentes locais da América Central e do Sul, integrando abordagens de modelagem para antecipar e combater os arbovírus emergentes. Os pesquisadores coordenadores, Nik Vasilakis e Kathryn Hanley tem colaboração estabelecida com ampla gama de parceiros nos EUA, Brasil e Panamá para cumprir a missão do CREATE-NEO.
|
|
Como uno de los diez Centros para la Investigación de Enfermedades Infecciosas Emergentes (https://creid-network.org/) financiados por los NIH, la Coordinación de Investigación sobre Enfermedades Infecciosas Emergentes en el Neotrópico (CREATE-NEO, https://www.utmb.edu/createneo/home/create-neo-home) proporciona una red ágil y flexible de sitios de vigilancia en América Central y del Sur junto con enfoques de modelado de vanguardia para anticipar y contrarrestar los arbovirus emergentes. Nik Vasilakis y Kathryn Hanley de Co-PI se asocian con una amplia gama de socios en los EE. UU., Brasil y Panamá para promulgar la misión de CREATE-NEO.
|
|
Forest edge landscape context affects mosquito community composition and risk of pathogen emergence. Hendy A, Fé NF, Pedrosa I, Girão A, Dos Santos TNF, Mendonça CR, Andes Júnior JT, Assunção FP, Costa ER, Sluydts V, Gordo M, Scarpassa VM, Buenemann M, de Lacerda MVG, Mourão MPG, Vasilakis N, Hanley KA.bioRxiv [Preprint]. 2024 May 3:2024.04.30.591911. doi: 10.1101/2024.04.30.591911. Garcia-Oliveira GF, Guimarães ACDS, Moreira GD, Costa TA, Arruda MS, de Mello ÉM, Silva MC, de Almeida MG, Hanley KA, Vasilakis N, Drumond BP.Viruses. 2023 Dec 23;16(1):31. doi: 10.3390/v16010031.
The Coordinating Research on Emerging Arboviral Threats Encompassing the Neotropics (CREATE-NEO). Vasilakis N, Hanley KA.Zoonoses (Burlingt). 2023;3(1):16. doi: 10.15212/zoonoses-2022-0047. Epub 2023 Apr 18. |
|
  |
 |
 |
|
| At the 2023 CREID meeting: Ben Althouse (NMSU and UW), Nik Vasilakis (UTMB), Lee Gherke (MIT), Kathy Hanley, Shannan Rossi (UTMB), Livia Sacchetto (FAMERP), Mauricio Nogueria (FAMERP), Betania Drumond (UFMG). | In the Pantanal for fieldwork: second to left: Daniel Aguiar (UFMG), Barbara Han (Cary Institute), Adrian Castellanos (Cary Institute) and Kathy Hanley, with UFMG research team. | |
|
|
||
 |
 |
|
| In the Darien province of Panama: Jean Paul Carrera (in sunglasses) and Team (Gorgas Institute) moving a freezer in Darien, Panama. | In the Darien province of Panama, Michaela Buenemann on a piragua. |